The Heart
- ATTR and AL are the main types of heart amyloidosis
- Overall, ATTRwt is the most common type of cardiac amyloidosis
- Note: ATTR is divided into ATTRwt (wild type) and ATTRv (inherited)
- Cardiac ATTRwt, AL and ATTRv have different clinical features, investigation findings and prognosis
- They can be thought of as three different types of cardiac amyloidosis
- Unfortunately, these differing patterns of presentation are not specific enough to differentiate between the cardiac amyloidosis types
- Overall, ATTRwt is the most common type of cardiac amyloidosis
- Cardiac amyloid typing requires a combination of the following essential tests;
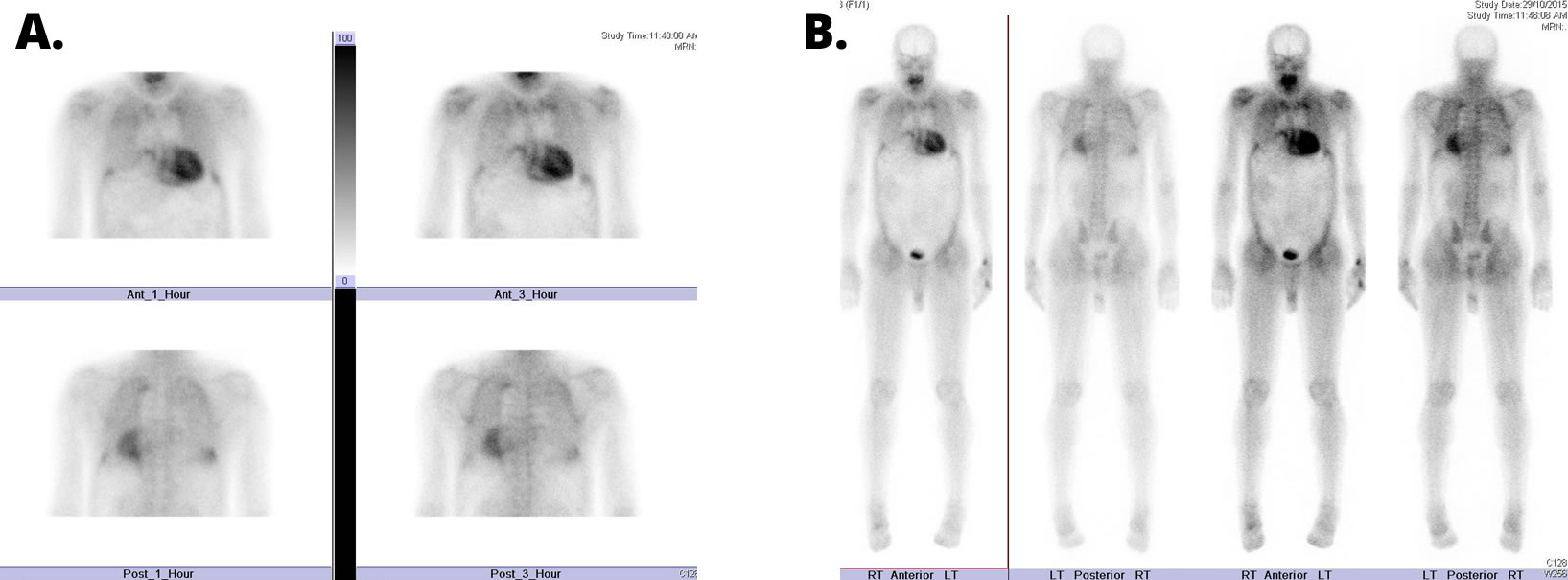
- Cardiac amyloid scintigraphy using specific bisphosphonate/bone tracers (for cardiac ATTR)
- Monoclonal gammopathy testing (for AL)
- This is required to screen for the presence of the plasma cell clone that creates AL
- This is also required when performing cardiac amyloid scintigraphy as the presence of a monoclonal gammopathy lowers the specificity of a positive scan result for cardiac ATTR
- as a minority of cardiac AL cases can also show cardiac amyloid scan uptake
- Tissue biopsy and anatomical pathology typing of amyloid deposits
- is required when there is a monoclonal gammopathy to differentiate between
- Cardiac ATTR with an unrelated benign non amyloid forming monoclonal gammopathy
- Cardiac AL caused by a plasma cell clone forming amyloidogenic monoclonal light chain
- is required when there is a monoclonal gammopathy to differentiate between
- A diagnostic algorithm to identify and then subtype cardiac amyloid is presented in the section “Cardiac Amyloidosis for Cardiologists”
- This part of the AAN website will discuss clinical features, cardiac test findings and the management of the most common cardiac amyloidosis types
- Cardiac amyloidosis is often a delayed diagnosis as clinical and test findings can mimic other more common diagnoses
- It is important to diagnose as early as possible as this can result in earlier institution of disease modifying treatment and longer survival
- The most common types of heart amyloid are ATTR (overwhelmingly ATTRwt subtype over the rarer ATTRv) and AL
- ATTR and AL account for over 95% of all cardiac amyloidosis1
- Overall, the most common type of cardiac amyloidosis is ATTRwt
- ATTR is subdivided into
- ATTRwt = wild type transthyretin amyloidosis where the ATTR is made up of physiologic “wild type” transthyretin
- ATTRv = inherited transthyretin amyloidosis where the “v” refers to a “variant” (See ATTR)
- ATTR is subdivided into
- Overall, the most common type of cardiac amyloidosis is ATTRwt
- ATTR and AL account for over 95% of all cardiac amyloidosis1
- Histologically ATTRwt amyloid is found in 10-25% of patients with HFpEF on autopsy studies2 and an even greater proportion of cases are detected in unselected cadaveric case series
- Thus ATTRwt is increasingly referred to as an age-related disorder
- The breakdown of the types of cardiac amyloid referred to AAN clinics is3;
- 63% ATTRwt
- 33% AL
- 3% ATTRv
- 1% “Other” ie AA, non TTR inherited amyloidosis such as apolipoprotein A1
- For ATTR, the most common cardiac amyloidosis, there is now a non-invasive way to diagnose this disorder as well as new disease modifying treatments
- ATTRwt, ATTRv and AL cardiac amyloid are different cardiac diseases4
- There are differences in the pathology, clinical presentation and investigation findings between ATTRwt and ATTRv and AL
- Unfortunately, these differences are not specific enough to subtype the amyloid and cannot be used to replace amyloid typing tests
- The tests that are used to type cardiac amyloidosis are listed below and are required in different combinations;
- Cardiac amyloid scintigraphy using Technetium labelled specific bisphosphonate/bone tracers (for cardiac ATTR)
- Monoclonal gammopathy testing (mandatory for all cases)
- This is required to screen for the presence of the plasma cell clone that creates AL
- This is also required when performing cardiac amyloid scintigraphy as the presence of a monoclonal gammopathy lowers the specificity of a positive scan result for cardiac ATTR
- This is because a minority of cardiac AL cases can also show uptake on cardiac amyloid DPD/PYP scintigraphy
- Hence biopsy and anatomical pathology typing is only required when there is a monoclonal gammopathy in order to differentiate between
- ATTR with an unrelated benign monoclonal gammopathy
- AL with deposits formed from amyloidogenic monoclonal light chain produced by a plasma cell clone
- The management for amyloid cardiomyopathy is distinctly different to that of other cardiomyopathies
- The guidelines for low EF and/or ischaemic cardiomyopathy are not applicable to the infiltrative/deposition disorder of amyloid cardiac amyloid
- Cardioembolic risk is higher in cardiac amyloidosis
- Anticoagulation should always be considered in atrial fibrillation regardless of CHADS-VASc score, and can be considered in cases of severe cardiac involvement without atrial fibrillation
- This part of the website will describe the clinical features, cardiac test findings and the cardiac support of those with cardiac amyloidosis
- A diagnostic algorithm for the identification and typing of cardiac amyloid is discussed in the Cardiac Amyloidosis for Cardiologists section
Amyloidosis and the Heart
Pathophysiology
KEY POINT
- Amyloid deposits in the myocardial extracellular interstitial spaces
- It is an infiltrative disorder with different pathology to cardiac muscle hypertrophic disorders
- Amyloid can eventually deposit in both ventricles and both atria (as well as other areas of the heart)
- The pattern of myocardial deposition differs between ATTR and AL
- Vascular amyloid is more common in AL
- In AL, amyloidogenic light chains are also probably directly cardio-toxic
- Amyloid deposits in the myocardial extracellular interstitial spaces separating and distorting the myocardial cells
- It deposits in the following areas;
- both ventricles
- the presence of right ventricular thickening can differentiate amyloid from processes that usually change the left ventricular parameters alone (such as hypertension)
- both atria
- the valves
- pericardium
- usually leading to small rather than large effusions
- coronary arteries
- classically in the small intramural vessels but not the epicardial coronaries
- both ventricles
- There are differences in the amyloid deposition pattern between the amyloid types
- The myocardial deposition pattern is
- subendocardial and diffuse in AL
- can be patchy and transmural in ATTRwt
- Vascular deposition
- Coronary artery involvement of the small intramural vessels is more common in AL than ATTR5
- The myocardial deposition pattern is
- Myocardial scarring and patchy fibrosis (typical of chronic ischaemic or other non-ischaemic cardiomyopathies) is not described in cardiac amyloidosis
- In cardiac AL disease, research also suggests that circulating AL light chains are directly cardiotoxic6
Clinical presentation
KEY POINT
- Cardiac amyloid deposition may remain symptom free for years (particularly with ATTRwt deposits)
- Cardiac disease usually presents with diastolic heart failure, arrhythmias or conduction abnormalities
- Amyloid related heart failure is usually classified under the heart failure syndrome “Heart Failure with Preserved Ejection Fraction” (HFpEF)
- although reduced ejection fraction can occur in end stage disease
- Few studies have been done in arrhythmias in cardiac amyloidosis
- Atrial Fibrillation is the most common arrhythmia in ATTRwt
- Ventricular arrythmias and sudden death is more common in AL than ATTRwt
- Syncope is also a common presentation but may be non-cardiac in nature and most commonly multifactorial and caused by postural hypotension
- Most commonly cardiac amyloid disease clinically presents with heart failure and/or arrythmias and conduction abnormalities
- A distinction should be made between cardiac amyloid deposition vs disease;
- ATTRwt cardiac deposition is often symptomless for years before causing disease but may still be detected incidentally on TTE or cardiac amyloid bone scan prior to the development of symptoms/disease
- A distinction should be made between cardiac amyloid deposition vs disease;
- The heart failure is typically diastolic in nature
- The heart failure is classified under the heterogenous heart failure syndrome “Heart Failure with Preserved Ejection Fraction” (HFpEF)
- Reduced systolic function is not a common presenting feature but is seen in endstage disease
- The arrythmias can be both bradyarrhythmias and tachyarrhythmias
- Few studies have been done on the electrophysiologic abnormalities and arrhythmias associated with cardiac amyloidosis
- Abnormal electrophysiologic function appears to be most common in the His-Purkinje system
- Progressive conduction system disease is common in ATTR
- Atrial Fibrillation is the most common arrythmia encountered in cardiac amyloidosis. It is present in:
- Ventricular arrythmias are more common in AL10
- Non sustained VT was detected in 27% AL in one holtermonitor study11 but in but only in n=1/20 in an implanted cardiac rhythm recorder study12
- Sustained VT is uncommon
- Sudden death is common in advanced cardiac AL
- The incidence of sudden death appears to be no different between those with or without non-sustained VT11
- Non sustained VT was detected in 27% AL in one holtermonitor study11 but in but only in n=1/20 in an implanted cardiac rhythm recorder study12
- Electromechanical Dissociation (EMD) or pulseless electrical activity (PEA)
- Syncope is common;
- Cardiac causes are;
- Hypotension from the following cardiac contributors
- Reduced stroke volume
- or reduced left ventricular volume from the restricted infiltrated left ventricle)
- Cardiac medications
- over diuresis, anti-hypertensives
- Reduced systolic function
- although this is only seen in end-stage cardiac disease)
- Reduced stroke volume
- Arrythmias: VT, bradycardias, heart block
- Hypotension from the following cardiac contributors
- Other organs involved by amyloidosis can also cause postural hypotension related syncope via
- autonomic dysfunction
- seen in ATTRv and AL (but not in ATTRwt)
- nephrotic syndrome with the osmotic reduction in intravascular volume
- autonomic dysfunction
- Cardiac causes are;
Heart Investigation Findings
- The following heart investigation categories will be discussed:
- Cardiac biomarkers
- Electrocardiogram
- Non-invasive imaging
- Transthoracic echocardiogram
- Cardiac magnetic resonance Imaging
- Cardiac amyloid bone scintigraphy
- Cardiac biopsy
Supportive Care of the Heart with Amyloidosis
Introduction
- The management of cardiac amyloidosis is divided into
- Disease modifying therapy, which is specific for each type of amyloidosis and this is further subdivided into
- Anti-synthesis therapies
- directed against the amyloid forming mechanism to reduce further amyloid production, deposition and cardiac failure
- Amyloid clearing therapies
- directed against the amyloid deposit subtype to induce resorption or clearance (these therapies are currently limited). However, if anti-synthesis therapies are successful a proportion of patients may endogenously clear cardiac amyloid TO ADD link to “What is Amyloidosis” section where this concept is discussed
- Disease modifying therapy is directed and targeted against the specific amyloid type and hence differs between ATTR vs AL
- Anti-synthesis therapies
- Supportive therapy, is discussed in this section of the website and mainly addresses
- Heart Failure
- Arrhythmias
- Syncope
- Disease modifying therapy, which is specific for each type of amyloidosis and this is further subdivided into
- Many of the usual generic guidelines for systolic heart failure and arrythmias are not always generalizable to patients with cardiac amyloidosis. This is due to the
- different pathophysiology of amyloid cardiomyopathy i.e. systolic heart failure guidelines for ischaemic heart disease are not applicable to the infiltrative disorder of amyloid
- toxicity/intolerance to many common medical approaches to these conditions
- Negative chronotropic agents are often less well tolerated as cardiac amyloid patients can be dependent on an increased heart rate for cardiac output and are often hypotensive
- There are often other organs affected by amyloidosis further limiting patient tolerance to cardiac therapies i.e
- autonomic neuropathy of ATTRv contributing to hypotension
- nephrotic syndrome of AL contributing to fluid retention
- Cardiac medical therapy is challenging and requires a cautious approach and fine balancing with constant review and adjustment
- As with other causes of diastolic dysfunction, there are no evidence-based supportive therapies that have been found to decrease mortality in cardiac amyloidosis patients, but their quality of life can often be improved significantly with informed and tailored choices in supportive therapies